Single-use plastic products could have a more useful and less polluting future thanks to a new technique that “upcycles” them into valuable lubricants and waxes. The technique, known as catalytic hydrogenolysis, uses platinum nanoparticles supported on tiny cubes of strontium titanate perovskite material to convert energy-rich polyethylene molecules into liquid hydrocarbons. The high-quality nature of these hydrocarbons means they could be employed in consumer products, potentially reducing plastic pollution in the environment.
Polyolefins such as polyethylene are widely used in single-use plastic products because the starting materials to make them are cheap and abundant. While these products are vital in some applications – sterile packaging for foods or medical devices, for example – they are being produced in ever-increasing amounts. Around 380 Mt of plastic materials are created worldwide each year, corresponding to roughly 7% of all crude oil and natural gas produced, and some analysts predict that plastic production could quadruple by 2050.
Of these materials, 75% are discarded after just a single use. Most single-use plastic waste ends up in landfills, in the environment, or in incinerators that produce greenhouse gases and toxic by-products as well as electricity. The plastic left in the environment does not easily degrade because of the very strong carbon-carbon bonds present, but instead breaks down into microplastic particles. In instances where it is recycled, current methods tend to produce lower-value materials with degraded properties – a phenomenon known as “downcycling”.
A vast and as-yet untapped resource
Despite this, some scientists view polyolefin waste as a vast and untapped resource for producing higher-grade chemicals and new materials. According to a US-based team led by Kenneth Poeppelmeier at Northwestern University, Aaron Sadow at Ames Lab and Iowa State University and Massimiliano Delferro at Argonne National Laboratory, a more efficient technology for extracting value from discarded polymers could save the equivalent of up to 3.5 billion barrels of crude oil each year. Selective catalytic processes that upcycle plastic waste into valuable products are thus sorely needed.
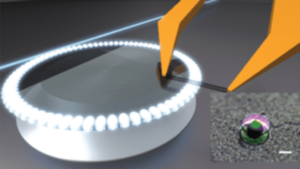
The researchers have now developed such a process. In their work, the researchers were able to react some of the strong C-C bonds of high-molecular-weight polyethylene with hydrogen converting the material into high-quality liquid hydrocarbons with a lower molecular weight and narrow distribution of between 200 to 1000 Da. Such liquids could be used as lubricating oils or as intermediates such as waxes that can be further processed into ingredients for everyday necessities such as detergents and cosmetics, say the researchers.

Composite chitin film could replace plastic packaging
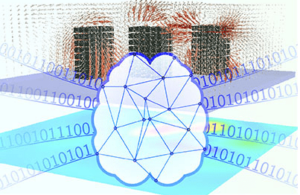
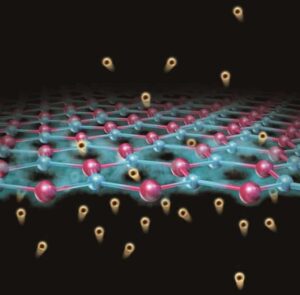
The catalyst they used consists of platinum nanoparticles just 2 nm in size deposited onto strontium titanate (SrTiO3) perovskite nanocubes (50-60 nm across) using a technique called atomic layer deposition. This technique, which was developed at Argonne National Laboratory, allows for precise control of the nanocubes. The researchers chose these materials because they are stable at high temperatures and pressures.
The catalyst cleaves the C-C bonds in the PE under moderate pressures of 170 psi H2 and temperatures of 300°C. The technique, detailed in ACS Central Science, also produces far less waste than conventional recycling methods, according to Poeppelmeier and colleagues.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://physicsworld.com/a/catalytic-technique-upcycles-single-use-plastic/
- :is
- :not
- :where
- $UP
- 200
- 90
- a
- Able
- abundant
- According
- across
- After
- All
- allows
- also
- amounts
- and
- applications
- ARE
- Argonne National Laboratory
- around
- AS
- At
- barrels
- BE
- because
- being
- between
- Billion
- Bonds
- breaks
- but
- by
- called
- CAN
- Catalyst
- central
- cheap
- chemicals
- chemistry
- chose
- colleagues
- consists
- consumer
- Consumer products
- control
- conventional
- convert
- converting
- Corresponding
- could
- created
- credit
- crude
- Crude oil
- Current
- DA
- deposited
- detailed
- detergents
- developed
- Devices
- distributed
- distribution
- does
- down
- each
- easily
- efficient
- electricity
- employed
- ends
- Environment
- EPA
- Equivalent
- ever-increasing
- everyday
- example
- far
- Film
- flexible
- foods
- For
- from
- further
- future
- GAS
- Have
- High
- high-quality
- http
- HTTPS
- hydrogen
- image
- in
- information
- instead
- into
- issue
- IT
- jpg
- just
- known
- laboratory
- layer
- Led
- left
- less
- Liquid
- lower
- make
- material
- materials
- max-width
- means
- medical
- medical devices
- methods
- moderate
- molecular
- more
- more efficient
- most
- MT
- National
- Natural
- Natural Gas
- Nature
- necessities
- needed
- New
- now
- of
- Oil
- oils
- on
- or
- P&E
- packaging
- phenomenon
- Physics
- Physics World
- plastic
- platinum
- plato
- Plato Data Intelligence
- PlatoData
- Pollution
- Polymers
- potentially
- precise
- predict
- present
- process
- processed
- processes
- produce
- Produced
- produces
- producing
- Production
- Products
- properties
- React
- recycling
- reducing
- replace
- researchers
- resource
- roughly
- Save
- say
- scientists
- selective
- single
- Size
- some
- stable
- Starting
- State
- strong
- such
- Supported
- team
- Technology
- than
- thanks
- that
- The
- their
- Them
- These
- they
- this
- thumbnail
- to
- true
- under
- untapped
- use
- used
- uses
- using
- Valuable
- value
- Vast
- very
- View
- vital
- was
- Waste
- weight
- WELL
- were
- which
- while
- widely
- with
- Work
- world
- worldwide
- wrap
- year
- zephyrnet